WELCOME TO HIBISCUS CONSULTING
We are integrity-based professionals from various technical, scientific, engineering, operational, and regulatory backgrounds with decades of experience in GMP-regulated industries such as innovative pharmaceuticals, biotechnology, Food, Nutraceuticals and medical device industries.
Hibiscus Consulting is a dynamic consulting firm specialized in providing QA, compliance, regulatory and project management services to the pharmaceutical, biotechnology, Food, Nutraceuticals and medical device industries.
Our consultants are seasoned professionals from various technical, scientific, and regulatory backgrounds with decades of collective experience in GMP-related industries. Our focused approach in providing real time and customized services to our clients has proven effective.
About us
About us
What we do
We are consultants to the regulated life science industries.We help clients achieve and maintain full compliance with India CDSCO, Health Canada, FDA, EMEA and WHO regulatory requirements. Our consultants facilitate you interface with various agencies to address quality & regulatory concerns.
In addition to providing strategic and management consulting services, we bring technical expertise in quality assurance, project management, validation and regulatory compliance.
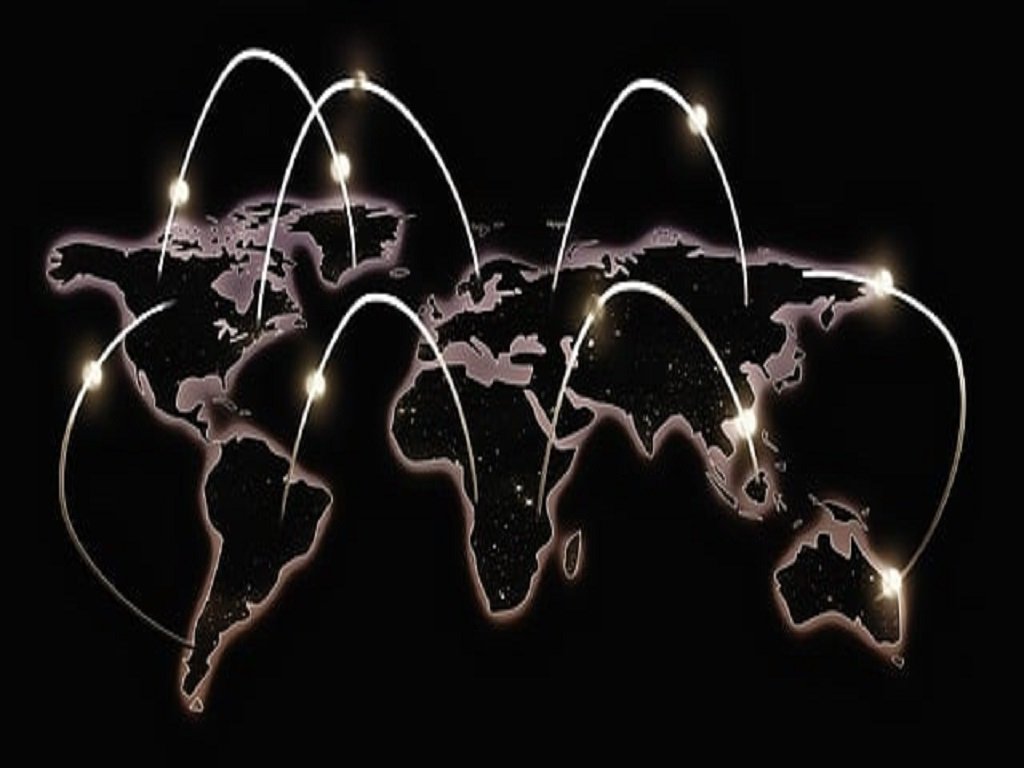
Where we work
Hibiscus offices are located in both south and north India. Further, Hibiscus strategically tied up with consultants and partners located in United States, Canada, UK to provide services to firms across the Americas, Europe and Asia. Our multicultural and multilingual professionals are uniquely positioned to optimize collaborations with your teams, no matter where you are located. Hibiscus Consulting enjoy strategic collaborations with quality auditors, consultants and professionals in key regions of the world allowing seamless project transition and team integration.
...
From our partner offices in UK, Canada and the United States, our consultants provide services to firms across the Americas, Europe and Asia. Our multicultural and multilingual professionals are uniquely positioned to optimize collaborations with your teams, no matter where you are located.
Hibiscus Consulting enjoy strategic collaborations with quality auditors, consultants, and professionals in key regions of the world allowing seamless project transition, and team integration.
Who we are
Hibiscus Consulting is a dynamic consulting firm specialized in providing QA, compliance, regulatory and project management services to the pharmaceutical, biotechnology, Food, Nutraceuticals and medical device industries.
Our consultants are seasoned professionals from various technical, scientific, and regulatory backgrounds with decades of collective experience in GMP-related industries. Our focused approach in providing real time and customized services to our clients has proven to be very effective.
Team
Check our Team
Clients
Pharma

Clients
Chinese Overseas Clients

Clients
Medical device

Hibiscus Consulting is the first empanelled agency for Andhra Pradesh based Govt.undertaking institution called Andhra Med Tech zone, to provide quality & regulatory services at Device firms.
Clients
Food & Nutraceuticals

Contact
Contact Us
Email :
pnbvarma@hibiscusconsult.net
pnbvarma@outlook.com
Call :
+91 7013715719
Office Locations
Visakhapatnam:
39-33- 146/1 MIG-74, PHASE-2, MADHAVADHARA VUDA COLONY, Visakhapatnam (Urban) PO: Marripalem, DIST: Visakhapatnam Andhra Pradesh - 530018
Haryana:
Signature Global The Millennia,Garauli Kalan Sector 37C Gurugram Haryana
Mumbai :
Office 1306, 13th floor Plot No.2 Sector no.14, Kamdhenu Commerz Kharghar navi Mumbai, Pin:-410210, Maharashtra INDIA
UK :
Headington Medical Technology Limited 3-4, Reliance Way, OX4 2FU, Oxford, England UK
USA :
22320 Foothill Bivd.#330 Hayward, California 94541 United states.
Canada :
6500 trana Canada Hwy, Suite 400 Pointe-Ciatre(Quebec) H9R0A5 CANADA
Europe:
V Ramesh, Skandinavische 3A, Edge 4 Berlin 10317